BUCCAL DRUG DELIVERY SYSTEM
(Mechanism of drug permeation
Methods of formulation and
evaluation)
SUBMITTED BY:
SADAF SALEEM
M.Pharm (Pharmaceutics)
Sem-I
SPER, JAMIA HAMDARD
CONTENTS
• Introduction
• General considerations
• Mechanism of permeation
• Formulation
• Methods of manufacture of mucoadhesive
buccal films/patches
• Basic components of drug delivery system
formulations
• Evaluation of formulations
• References
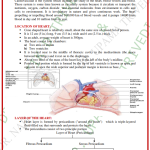
INTRODUCTION
• Delivery of drugs through buccal mucosa of the
oral cavity is known as buccal drug delivery
system(BDDS).
• The product is placed in between the upper
gingiva(gums) and cheek to provide local and
systemic effects.
• The main impediment to the use of many
hydrophilic macromolecular drugs as potential
therapeutic agents is their inadequate and
erratic oral absorption.
Fig.- A TRANSDERMAL PATCH APPLIED TO THE BUCCAL
MUCOSA
Fig.- A TRANSDERMAL PATCH APPLIED TO THE
BUCCAL MUCOSA
GENERAL CONSIDERATIONS
Drugs can be absorbed from the oral cavity
through the oral mucosa.
Rapid absorption from buccal route is observed
because of the thin mucous membrane and rich
blood supply.
The larger molecules are poorly absorbed.
Buccal dosage forms are typically short acting
because of limited contact time between the
dosage form and the oral mucosa.
Since sublingual administration of drugs
interferes with eating, drinking, and talking, this
route is generally considered unsuitable for
prolonged administration.
The duration of buccal drug administration can be
prolonged with saliva-activated adhesive troches.
MECHANISM OF DRUG
PERMEATION
• There are two routes potentially
involved in drug permeation across
epithelial membranes:
– The transcellular route
– The paracellular route
Fig- mechanism of drug permeation
Transcellular route
• For lipophilic compounds.
• The drugs permeate by passing through
the cells of the membrane.
• Lipophilic drugs have very high
permeability across the epithelial cell
membrane.
• Drugs movement through this pathway(J)
is given by:
Paracellular route
• For hydrophilic compounds.
• The drugs permeate by passing through
the intercellular spaces.
• Lipophilic drugs do not pass through
these spaces.
• Drugs movement through this pathway(J)
can be given by:
DESIGN OF BUCCAL
DOSAGE FORM
• Matrix type :
• The Buccal patch designed in a matrix
configuration contains drug, adhesive, and
additives mixed together.
• Reservoir type:
• The buccal patch designed in a reservoir system
contains a cavity for the drug and additives
separate from the adhesive.
Design of Buccal Dosage Form:
FORMULATIONS
Buccal drug
delivery system
Non attached Bioadhesive
drug delivery drug delivery
system system
• Non attached drug delivery system
This includes:
-Fast dissolving tablet dosage forms
-Chewing gum formulations
-Micro-porous hollow fibers
The local physiological environment greatly
affects the nonattached drug delivery system,
e.g. the presence of saliva
the intake of foods and liquids.
• Bio-adhesive drug delivery system
These are the formulations that are designed
in such a manner so as to adhere to the
mucosa of the oral cavity.
They are likely to get affected by local
physiological factors as:
-presence of saliva
-Intake of food and liquids
but not to a great extent.
Bio-adhesive drug delivery system can be
classified as:
1) SOLID BUCCAL ADHESIVE DOSAGE FORMS:
• Tablets
• Micro particles (microspheres, microcapsules)
• Wafers
• Lozenges
2) LIQUID BUCCAL ADHESIVE DOSAGE FORMS:
• Viscous liquids
3) SEMI SOLID BUCCAL ADHESIVE DOSAGE FORMS:
• Gels
• Buccal Patches
• Buccal films
Buccal drug delivery system
Bio-adhesive DDS Non-attached DDS
SOLID BUCCAL ADHESIVE LIQUID BUCCAL ADHESIVE SEMI SOLID BUCCAL
DOSAGE FORMS DOSAGE FORMS ADHESIVE DOSAGE FORMS
VISCOUS
TABLETS GELS
LIQUIDS
MICROPARTI
PATCHES
CLES
WAFERS FILMS
LOZENGES
CLASSIFICATION OF BUCCAL DRUG DELIVERY SYSTEM
SOLID BUCCAL ADHESIVE DOSAGE
FORMS:
• Buccal tablets
Buccal tablets are small, flat and oval in shape
with a diameter of approximately 5–8 mm.
The direct compression technique is most
widely used for preparation of buccal tablets,
techniques like wet granulation can also be
employed.
Slide Title
BUCCAL TABLET
• Microparticles (microcapsules and microspheres)
The local irritation caused by microspheres or
microcapsules or microparticles at the site of adhesion
is less and are comfortable with sensation of a foreign
object within the oral cavity.
They are more palatable as compared to
the buccal tablets.
• Wafers
Wafer is a drug delivery system with surface layers
possessing adhesive properties.
• Lozenges
Bioadhesive lozenge offers prolonged drug release with
improved patient compliance, thus avoiding multiple
daily dose.
LIQUID BUCCAL ADHESIVE DOSAGE
FORMS:
• Viscous liquids
Liquids used to coat buccal surface are
viscous and serve as either protective agents
or as drug vehicles for delivery of drug on to
the mucosal surface.
Pharmaceutically acceptable polymers are
used to improve the viscosity of products to
aid their maintenance in the oral cavity.
Lubrication can be provided by treating dry
mouth with artificial saliva solutions and to
retain the drug on mucosal surfaces.
SEMI SOLID BUCCAL ADHESIVE DOSAGE
FORMS:
• Gels
Bioadhesive polymers forming gels, which
form cross linked polyacrylic acid, in which
mucosal surfaces can be fixed are used to
provide the release in control manner for
extensive period of time and drug at the
absorption site.
Bioadhesive polymers forming gels are of
limited use for drugs with narrow therapeutic
window due to their inability to deliver a
measured dose of drug to the site.
• Buccal patches
Patches are laminates consists of drug
containing reservoir layer and an
impermeable backing layer.
Drug is released in a controlled manner from
the drug-containing reservoir layer, and a
bio-adhesive surface for mucosal
attachment.
Patches have unique characteristics,
including relatively rapid onset of drug
delivery, sustained drug release and rapid
decline in serum drug concentration when
the patch is removed.
• Buccal films
These are the most recently developed dosage
form which meant for buccal administration.
Buccal films have more flexibility and comfort
when compared with adhesive tablets. So,
buccal films are preferred instead of adhesive
tablets.
An ideal film should be soft, elastic, flexible
and posses adequate strength to withstand
breakage due to stress from mouth
movements.
METHODS OF MANUFACTURE OF
MUCOADHESIVE BUCCAL
FILMS/PATCHES
• The main manufacturing processes involved in
mucoadhesive buccal films are as follows:
1. Solvent casting
2. Hot-melt extrusion
3. Direct milling
SOLVENT CASTING METHOD
The drug and excipients is dissolved in
appropriate solvent and water soluble
polymers are dissolved in water and these
solutions are stirred and at last casted into the
petri plate and dried.
The solvent is evaporated by casting the
solution of the drug and polymer onto a
backing layer sheet and the patches were
punched in intermediate sheet.
The steps involved in solvent casting method are:
API and other excipients are dissolved in appropriate
solvent to form a clear viscous solution
The formed solutions are mixed
Solution is cast as a film and allowed to dry
Film is coated.
HOT-MELT EXTRUSION
In hot melt extrusion blend of
pharmaceutical ingredients is molten.
Blend is then forced through an orifice to
yield a more homogeneous material in
different shapes such as granules, tablets, or
films.
The steps involved in hot melt extrusion method are:
In dry state drug is mixed with carriers
Extrude via heating melts the mixture
The mass is cast in the films by the die.
DRY MILLING
Drug and excipients are mixed by kneading,
usually without the presence of any liquids.
After the mixing process, material is rolled
on a release liner until the desired thickness
is achieved.
The backing material is then laminated.
The steps involved in dry milling method are:
API and excepients are blended by direct
milling
Blended mixture is rolled with help of a
roller
Material is laminated and film collected
BASIC COMPONENTS OF DRUG
DELIVERY SYSTEM FORMULATIONS
The basic components of buccal drug delivery
system are:
Drug substance
Bio-adhesive polymers
Backing membrane
Penetration enhancers
Drug substance:
The drug should have following characteristics:
• The conventional single dose of the drug
should be small.
• The drugs should have biological half-life
between 2-8 hours.
• Tmax of the drug shows wider-fluctuations or
higher values when given orally.
• Drug may exhibit first pass effect or pre-
systemic drug elimination when given orally.
• The drug absorption should be passive when
given orally.
LIST OF APIs THAT CAN BE DELIVERED THROUGH BDDS
Bio-adhesive polymers:
They control the duration of release of drugs.
An ideal polymer for buccal drug delivery systems
should have following Characteristics:
• It should be inert and compatible with the
environment.
• The polymer and its degradation products should be
non-toxic absorbable from the mucous layer.
• It should adhere quickly to moist tissue surface and
should possess some site specificity.
• It must not decompose on storage or during the
shelf life of the dosage form.
• It should be easily available in the market and
economical.
• It should allow easy incorporation of drug in to the
formulation.
Backing Membrane:
• It plays a major role in the attachment of bio
adhesive devices to the mucus membrane.
• The materials used as backing membrane
should be inert, and impermeable to the drug
and penetration enhancer.
• Eg.-carbopol, magnesium stearate, HPMC, HPC,
CMC, polycarbophil
Penetration Enhancers:
• They are used to improve the release of the
drug.
• They aid in the systemic delivery of the drug by
allowing the drug to penetrate more readily into
the viable tissues.
• Eg.-Sodium lauryl sulphate, CPC, Polysorbate 80,
Laureth 9, Sodium Fusidate, Sodium
glycocholate, Dimethyl formamide
LIST OF PERMEATION ENHANCERS
Bioadhesives:
• The ideal bioadhesive should have the
following characters:
• It should not produce any residue on mucosa
layer.
• It should be inert and compatible with
biological environment.
• It should adhere to the mucus membrane
aggressively.
• It should preferably form a strong non-covalent
bond with mucin/ epithelial cell surface.
EVALUATION OF FORMULATIONS
1) Disintegration time
Slide frame method: Film on slide + drop of
water. Note the time when a hole develops in
film.
Petri dish method: Film in petri plate + 2ml of
water. Note the time till film dissolves in it.
2) Residence time
Performed using IP disintegration apparatus.
Temperature of 37 ± 2°C using 900 ml of the
disintegration medium.
Portion of the rat intestinal mucosa, each of 3
cm length, is glued to the glass piece surface.
A piece of formulation is attached to the
mucosa.
The time required for complete
detachment of the film from the mucosal
surface is to be noted.
3) Muco-adhesive strengths
Different forces evaluated in muco-adhesion tests
• SHEAR STRENGTH (for various polymers):
Measures the force required to separate two
polymer coated glass slides.
Fig.- Apparatus
• MUCO-ADHESION TEST:
The force required to detach the mucoadhesive
film from the mucosal surface
A modified balance method is used.
The mucosa was attached to a dry petri dish
surface and was moistened with a few drops of
simulated saliva.
The difference in weight was taken as mucoadhesive
strength.
• Mucoadhesive force = (Mucoadhesive strength
(g)/1000) x
acceleration due to
gravity (Kg/m/s)
(9.8 m/s-1)
Slide Title
• TEXTURE ANALZER:
Force required to remove the formulation
from a model membrane is measured.
4) Tensile Strength:
It is defined as the resistance of the material to
a force tending to tear it.
It was determined using an Instron universal
testing instrument.
Films are held between two clamps and were
pulled by the top clamp.
The force and elongation were measured when
the film broke.
It is given by the following equation:
Tensile strength = Force at break (N) / Cross-
sectional area of
film (mm2).
INSTRON UNIVERSAL TESTING
EQUIPMENT
5) Percent Elongation Break:
It is a measurement of the maximum
deformation the film can undergo before
tearing apart.
It is calculated using the following equation:
Elongation at break = Increase in length of
break / Initial film length x 100
OTHER TESTS
Film Weight and Thickness
The weight of each film was measured using a
digital balance among the three films of every
formulation and the average weight was
calculated.
The thickness of each film was measured using a
micrometer screw gauge at different points of the
film and the average was calculated.
Folding Endurance
It is done by repeatedly folding one film at the
same place till it broke or folded up to 300 times
manually.
The number of times the film could be folded at
the same place without breaking gives the value of
folding endurance.
Surface pH
Three films of each formulation to swell for two
hours on an agar plate surface.
pH was measured by means of pH paper.
Swelling Index
The films were weighed individually and placed on
the surface of an agar plate kept in an incubator
maintained at 37±0.2°c and were allowed to swell.
An increase in the weight of the film was noted
inregular intervals of time and the weight was
calculated.
Percent Swelling (%S) = (X t – X o /X o ) x 100
Where,
Xt = the weight of the swollen film after time t
Xo = the initial film weight at zero time.
Moisture Content
films weighed individually and kept in a
desiccator containing calcium chloride at
room temperature for 24 h.
After a specified interval, the films are to be
weighed again until they show an unvarying
weight.
The % moisture content is calculated:
% moisture content = (Initial weight – Final
weight/initial weight) x 100